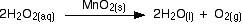|
|
|
Архитектура Астрономия Аудит Биология Ботаника Бухгалтерский учёт Войное дело Генетика География Геология Дизайн Искусство История Кино Кулинария Культура Литература Математика Медицина Металлургия Мифология Музыка Психология Религия Спорт Строительство Техника Транспорт Туризм Усадьба Физика Фотография Химия Экология Электричество Электроника Энергетика |
Zinc and hydrochloric acid
In the lab, zinc granules react fairly slowly with dilute hydrochloric acid, but much faster if the acid is concentrated.
The catalytic decomposition of hydrogen peroxide Solid manganese(IV) oxide is often used as a catalyst in this reaction. Oxygen is given off much faster if the hydrogen peroxide is concentrated than if it is dilute.
The reaction between sodium thiosulphate solution and hydrochloric acid This is a reaction which is often used to explore the relationship between concentration and rate of reaction in introductory courses (like GCSE). When a dilute acid is added to sodium thiosulphate solution, a pale yellow precipitate of sulphur is formed.
As the sodium thiosulphate solution is diluted more and more, the precipitate takes longer and longer to form.
The explanation Cases where changing the concentration affects the rate of the reaction This is the common case, and is easily explained. Collisions involving two particles The same argument applies whether the reaction involves collision between two different particles or two of the same particle. In order for any reaction to happen, those particles must first collide. This is true whether both particles are in solution, or whether one is in solution and the other a solid. If the concentration is higher, the chances of collision are greater.
Reactions involving only one particle If a reaction only involves a single particle splitting up in some way, then the number of collisions is irrelevant. What matters now is how many of the particles have enough energy to react at any one time. Note: If you aren't sure about this, then read the page about collision theory and activation energy before you go on. Use the BACK button on your browser to return to this page. Suppose that at any one time 1 in a million particles have enough energy to equal or exceed the activation energy. If you had 100 million particles, 100 of them would react. If you had 200 million particles in the same volume, 200 of them would now react. The rate of reaction has doubled by doubling the concentration.
Cases where changing the concentration doesn't affect the rate of the reaction At first glance this seems very surprising!
Поиск по сайту: |